Characterization of Atherosclerotic
Plaque Components by AFM
Jacques Ohayon, PhD and Philippe Tracqui, PhD
Laboratory
TIMC-IMAG/DyCTiM, UJF, CNRS UMR 5525, In3S
Faculty of Medicine of Grenoble - 38706 La Tronche Cedex, France
Email: Jacques.Ohayon@imag.fr
Mapping
elasticity moduli of atherosclerotic plaque in situ via atomic force microscopy
- Journal of structural Biology, 174(1):115-23, 2011 - Several studies have suggested that evolving
mechanical stresses and strains drive atherosclerotic plaque development and
vulnerability. Especially, stress distribution in the plaque fibrous capsule is
an important determinant for the risk of vulnerable plaque rupture.
Knowledge of the stiffness of atherosclerotic plaque components is
therefore of critical importance. In this work, force mapping experiments using
atomic force microscopy (AFM) were conducted in apolipoprotein E-deficient (ApoE−/−)
mouse, which represents the most widely used experimental model for
studying mechanisms underlying the development of atherosclerotic lesions. To obtain the elastic material
properties of fibrous caps and lipidic cores of atherosclerotic plaques, serial
cross-sections of aortic arch lesions were probed at different sites.
Atherosclerotic plaque sub-structures were subdivided into cellular fibrotic,
hypocellular fibrotic and lipidic rich areas according to histological
staining. Hertz’s contact mechanics were used to determine elasticity (Young’s)
moduli that were related to the underlying histological plaque structure.
Cellular fibrotic regions exhibit a mean Young modulus of 10.4 ±
5.7 kPa. Hypocellular fibrous caps were almost six-times stiffer, with average
modulus value of 59.4 ± 47.4 kPa, locally rising up to ~ 250
kPa. Lipid rich areas exhibit a rather large range of Young’s moduli, with
average value of 5.5 ± 3.5 kPa. Such precise quantification of
plaque stiffness heterogeneity will allow investigators to have prospectively a
better monitoring of atherosclerotic disease evolution, including arterial wall
remodeling and plaque rupture, in response to mechanical constraints imposed by
vascular shear stress and blood pressure.
|
|
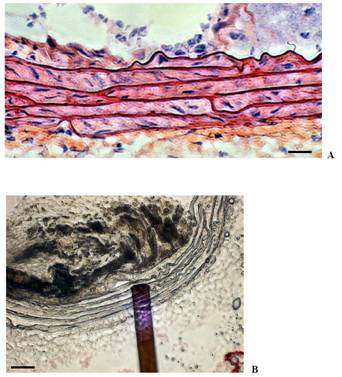
Fig. 1 : Mechanical
properties of arterial wall. A. Ultrastructure of arterial wall
revealed by HES staining, exhibiting the regular organization of elastin
sheets (scale bar 15 mm). B. Probing the stiffness of elastic
lamina by AFM force spectroscopy (scale bar 50 mm). |
Selected
Publications
• Ohayon J, Mesnier N, Broisat A, Toczek J, Riou
L, Tracqui P. Elucidating atherosclertic vulnerable plaque rupture by modeling
cross substitution of ApoE-/- mouse and human plaque component stiffnesses. Biomechanics
and Modeling in Mechanobiology. (in press), 2011.
• Ohayon J, Gharib AM, Garcia A,
Heroux J, Yazdani SK, Malvè M, Tracqui P, Martinez MA,
Doblare M, Finet G, Pettigrew RI. Is arterial wall-strain stiffening
and additional process responsible for atherosclerosis in coronary bifurcations? in
vivo Study Based on
• Broisat A,
Toczek J, Mesnier N, Tracqui P, Ghezzi C, Ohayon
J, Riou L. Assessing the low levels of mechanical stress in aortic
atherosclerosis lesions from ApoE-/-mouse . Arterioscler Thromb Vasc Biol.
31(5):1007-10, 2011.
• Tracqui P,
Broisat A, Toczek J, Mesnier N, Ohayon J,
Riou L. Mapping elasticity moduli of atherosclerotic plaque in situ via atomic
force microscopy . Journal of structural Biology 174(1):115-23,
2011.
• Heroux J, Gharib
AM,
• Soloperto G, Keenan NG, Sheppard MN, Ohayon
J, Wood N, Pennell DJ, Mohiaddin RH, Xu XY. A combined imaging,
computational and histological analysis of a ruptured carotid plaque. Artery
Research, 4(2):59-65, 2010.
• Le Floc'h S,
Cloutier G, Finet G, Tracqui P, Pettigrew RI, Ohayon J. On the
potential of a new IVUS elasticity modulus imaging approach for detecting
vulnerable atherosclerotic coronary plaques: in vitro vessel phantom study. Phys.
Med. Biol., 55:5701-5721, 2010.
• Finet G., Huo Y,
Riouffol G, Ohayon J, Guerin P,
Kassab GS. Structure-function relation in the
coronary artery tree: from fluid dynamics to arterial bifurcations. EuroIntervention,
6:J10-J15, 2010.
• Le Floc'h S, Ohayon J, Tracqui P, Finet G, Gharib AM,
Maurice R, Cloutier G, Pettigrew RI. Vulnerable Atherosclerotic Plaque
Elasticity Reconstruction Based on a Segmentation-Driven Optimization Procedure
Using Strain Measurements: Theoretical Framework. IEEE
Trans Med Imaging, 28(7):1126-37, 2009.
• Kotys MS,
Herzka DA, Vonken EJ, Ohayon J,
Heroux J, Gharib AM, Stuber M, Pettigrew RI. Profile order and time-dependent
artifacts in contrast-enhanced coronary MR angiography at 3T: origin and
prevention. Magn Reson Med., 62(2):292-9, 2009.
• Eskandari H,
Salcudean SE, Rohling R, Ohayon J.
Viscoelastic characterization of soft tissue from dynamic finite element
models. Physics in Medicine and Biology, 53(22):6569-90, 2008.
• Ohayon J, Finet G, Gharib AM, Herzka DA,
Tracqui P, Heroux J, Rioufol G, Kotys MS, Elagha A, Pettigrew RI. Necrotic core
thickness asnd positive arterial remodeling index: emergent biomechanical
factors for evaluating the risk of plaque rupture. Am
J Physiol Heart Circ Physiol., 295(2):H717-27, 2008.
• Ohayon J, Dubreuil O, Tracqui P, Le Floc'h S,
Rioufol G, Chalabreysse L, Thivolet F,
• Boudou T., Ohayon J., Arntz Y., Finet G., Picart C.,
Tracqui P. An extended modeling of the micropipette aspiration experiment for
the characterization of the Young’s modulus and Poisson’s ratio of adherent
thin biological samples: Numerical and experimental studies. Journal of Biomechanics,
39:1677-85, 2006.
• Boudou T., Ohayon J., Picart C., Tracqui P. Characterization of the Young’s modulus and Poisson’s ratio of polyacrylamide gels using micropipette aspiration technique. Biorheology, 43(6): 721-8, 2006.