Residual Stress/Strain Distributions in Mouse and Human Vulnerable Atherosclerotic Plaques
Jacques Ohayon, PhD
Laboratory TIMC-IMAG/DyCTiM, UJF,
CNRS UMR 5525, In3S
Faculty of Medicine of Grenoble - 38706 La Tronche Cedex, France
Email: Jacques.Ohayon@imag.fr
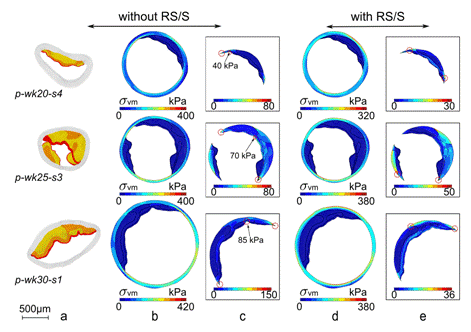
Assessing the low levels of mechanical stress in aortic atherosclerosis lesions from ApoE-/-mouse - Arterioscler Thromb Vasc Biol. 31(5):1007-10, 2011 - Despite the fact that mechanical stresses are well recognized as key determinants for atherosclerotic plaque rupture, very little is known about stress amplitude and distribution in atherosclerotic lesions, even in the standard apoE-/- mouse model of atherosclerosis. Our objectives were to combine immunohistology, atomic force microscopy (AFM) measurements and finite element computational analysis for the accurate quantification of stress amplitude and distribution in apoE-/- mouse aortic atherosclerotic lesions. Residual stresses and strains (RSS) were released by radially cutting aortic arch segments from 7- to 30 weeks-old pathological apoE-/- (n=25) and healthy control mice (n=20). Immunohistology, AFM, and biomechanical modelling taking into account regional RSS were performed. Maximum stress values were observed in the normal arterial wall (276 ± 71 kPa), whereas low values(<20kPa) were observed in all plaque areas. Stress distribution was not correlated to macrophage infiltration. Low mechanical stress amplitude was observed in apoE-/- mice aortic atherosclerotic lesions. This original study provides a basis for further investigations aimed at determining whether low stress levels are responsible for the apparently higher stability of murine aortic atherosclerotic lesions.
|
|
Fig. 1: Computation of the in vivo stress distributions in mouse atherosclerotic arterial wall submitted to physiological internal pressure of 14.5kPa and without considering residual stress/strain RS/S (columns b and c) or taking into account RS/S (columns d and e). Column a: close unloaded configurations of 3 atherosclerotic lesions (pathological samples # 4, 3 and 1 for mice of 20, 25 and 30 weeks, respectively). (yellow: LiRi regions, orange: CeFb regions, red: HyFb regions, white: arterial wall). Rectangular frames highlight intraplaque stress distributions only. |
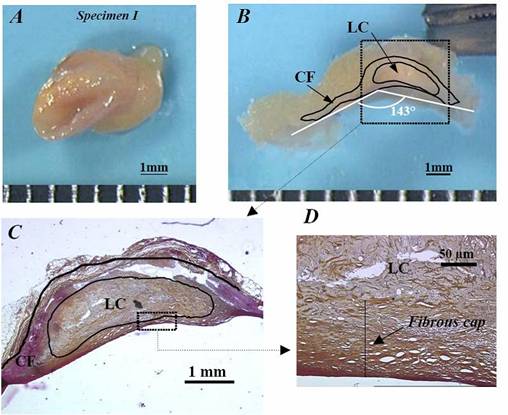
Influence of Residual Stress/Strain on the Biomechanical Stability of Vulnerable Coronary Plaques: Potential Impact for Evaluating the Risk of Plaque Rupture - Am J Physiol Heart Circ Physiol., 293(3):H1987-96, 2007 - In a vulnerable plaque (VP), rupture often occurs at a site of high stress within the cap. It is also known that vessels do not become free of stress when all external loads are removed. Previous studies have shown that such residual stress/strain (RS/S) tends to make the stress distribution more uniform throughout the media of a normal artery. However, the influence of RS/S on the wall stress distribution in pathological coronaries remains unclear. The aim of this study was to investigate the effects of RS/S on the biomechanical stability of VPs. RS/S patterns were studied ex vivo in six human vulnerable coronary plaque samples. Since the existence of RS/S can only be assessed by releasing it, the opening angle technique was the experimental approach used to study the geometrical opening configurations of the diseased arteries, producing an arterial wall in a near-zero stress state. Reciprocally, these opening geometries were used in finite element simulations to reconstruct the RS/S distributions in closed arteries. It was found that the RS/S: i) is not negligible, ii) dramatically affects the physiological peak stress amplitude in the thin fibrous cap, iii) spotlights some new high stress areas, and iv) could be a landmark of the lipid core’s developmental process within a VP. This study demonstrates that plaque rupture is not to be viewed as a consequence of intravascular pressure alone, but rather of a subtle combination of external loading and intraplaque RS/S.
|
|
|
Fig. 2: Description of the protocol performed on human specimen I to obtain the geometry of the macroscopic zero-stress configuration. A) isolated left anterior descending coronary sample of 4mm length. B) zero-stress configuration obtained 3 hours after radial sectioning of the healthy arc. The opening angle was measured as 143°. C) macroscopic histological observation of the same sample enabling the contours of the plaque constituents to be defined. D) microscopic histological view of the thin fibrous cap, from which the cap thickness CTh was approximated (CTh close to 94 µm). LC= lipid core; CF: cellular fibrosis. |
Selected Publications
• Ohayon J, Mesnier N, Broisat A, Toczek J, Riou L, Tracqui P. Elucidating atherosclertic vulnerable plaque rupture by modeling cross substitution of ApoE-/- mouse and human plaque component stiffnesses. Biomechanics and Modeling in Mechanobiology. (in press), 2011.
• Ohayon J, Gharib AM, Garcia A, Heroux J, Yazdani SK, Malvč M, Tracqui P, Martinez MA, Doblare M, Finet G, Pettigrew RI. Is arterial wall-strain stiffening and additional process responsible for atherosclerosis in coronary bifurcations? in vivo Study Based on Dynamic CT and MRI. Am J Physiol Heart Circ Physiol. 301(3):H1097-106, 2011.
• Broisat A, Toczek J, Mesnier N, Tracqui P, Ghezzi C, Ohayon J, Riou L. Assessing the low levels of mechanical stress in aortic atherosclerosis lesions from ApoE-/-mouse . Arterioscler Thromb Vasc Biol. 31(5):1007-10, 2011.
• Tracqui P, Broisat A, Toczek J, Mesnier N, Ohayon J, Riou L. Mapping elasticity moduli of atherosclerotic plaque in situ via atomic force microscopy . Journal of structural Biology 174(1):115-23, 2011.
• Heroux J, Gharib AM, Danthi NS, Cecchini S, Ohayon J, Pettigrew RI. High Affinity avb3 Integrin Targeted Optical Probe as a New Imaging Biomarker for Early Atherosclerosis: Initial Studies in Watanabe Rabbits. Mol Imaging Biol., 12(1):2-8, 2010.
• Soloperto G, Keenan NG, Sheppard MN, Ohayon J, Wood N, Pennell DJ, Mohiaddin RH, Xu XY. A combined imaging, computational and histological analysis of a ruptured carotid plaque. Artery Research, 4(2):59-65, 2010.
• Le Floc'h S, Cloutier G, Finet G, Tracqui P, Pettigrew RI, Ohayon J. On the potential of a new IVUS elasticity modulus imaging approach for detecting vulnerable atherosclerotic coronary plaques: in vitro vessel phantom study. Phys. Med. Biol., 55:5701-5721, 2010.
• Finet G., Huo Y, Riouffol G, Ohayon J, Guerin P, Kassab GS. Structure-function relation in the coronary artery tree: from fluid dynamics to arterial bifurcations. EuroIntervention, 6:J10-J15, 2010.
• Le Floc'h S, Ohayon J, Tracqui P, Finet G, Gharib AM, Maurice R, Cloutier G, Pettigrew RI. Vulnerable Atherosclerotic Plaque Elasticity Reconstruction Based on a Segmentation-Driven Optimization Procedure Using Strain Measurements: Theoretical Framework. IEEE Trans Med Imaging, 28(7):1126-37, 2009.
• Kotys MS, Herzka DA, Vonken EJ, Ohayon J, Heroux J, Gharib AM, Stuber M, Pettigrew RI. Profile order and time-dependent artifacts in contrast-enhanced coronary MR angiography at 3T: origin and prevention. Magn Reson Med., 62(2):292-9, 2009.
• Eskandari H, Salcudean SE, Rohling R, Ohayon J. Viscoelastic characterization of soft tissue from dynamic finite element models. Physics in Medicine and Biology, 53(22):6569-90, 2008.
• Ohayon J, Finet G, Gharib AM, Herzka DA, Tracqui P, Heroux J, Rioufol G, Kotys MS, Elagha A, Pettigrew RI. Necrotic core thickness asnd positive arterial remodeling index: emergent biomechanical factors for evaluating the risk of plaque rupture. Am J Physiol Heart Circ Physiol., 295(2):H717-27, 2008.
• Ohayon J, Dubreuil O, Tracqui P, Le Floc'h S, Rioufol G, Chalabreysse L, Thivolet F, Pettigrew RI, Finet G. Influence of residual stress/strain on the biomechanical stability of vulnerable coronary plaques: potential impact for evaluating the risk of plaque rupture. Am J Physiol Heart Circ Physiol., 293(3):H1987-96, 2007.
• Boudou T., Ohayon J., Arntz Y., Finet G., Picart C., Tracqui P. An extended modeling of the micropipette aspiration experiment for the characterization of the Young’s modulus and Poisson’s ratio of adherent thin biological samples: Numerical and experimental studies. Journal of Biomechanics, 39:1677-85, 2006.
• Boudou T., Ohayon J., Picart C., Tracqui P. Characterization of the Young’s modulus and Poisson’s ratio of polyacrylamide gels using micropipette aspiration technique. Biorheology, 43(6): 721-8, 2006.