Atherosclerotic
Plaque Rupture in
Humans and Mice
Jacques
Ohayon, PhD
Laboratory
TIMC-IMAG/DyCTiM, UJF,
CNRS UMR 5525, In3S
Faculty of Medicine of
Grenoble
- 38706 La Tronche Cedex ,
France
Email: Jacques.Ohayon@imag.fr
Elucidating
atherosclertic
vulnerable plaque rupture by modeling
cross substitution of ApoE-/-
mouse and human plaque component stiffnesses - Biomechanics and Modeling in Mechanobiology. (in press), 2011 - The structure of mouse atherosclerotic lesions
may differ from that of humans, and mouse atherosclerotic plaque do not
rupture
except in some specific locations such as the brachiocephalic
artery. Recently, our group was the first to observe that the
amplitudes of in
vivo stresses in ApoE-/-
mouse aortic
atherosclerotic lesions were much lower and differed from those found
in a
previous work performed on human lesions. In this previous preliminary
work, we
hypothesized that the plaque mechanical properties (MP) may in turn be
responsible
for such species differences. However, the limited number of human
samples used
in our previous comparative study was relevant but not sufficient to
broadly
validate such hypothesis. Therefore, in this study, we propose an original finite element strategy that
reconstructs the in vivo
stress/strain (IVS/S) distributions in ApoE-/-
artherosclerotic
vessel based on cross substitution
of ApoE-/-
mouse and human plaque
components stiffnesses and
including residual stress/strain (RS/S). Our results: 1)
showed that
including RS/S decreases by a factor 2 the amplitude of maximal IVS/S,
and more
importantly 2) demonstrated that the
MP of the ApoE-/-
plaque constituents are mainly
responsible for the low level - compared to human - of intraplaque
stress in ApoE-/-
mouse aortic
atherosclerotic lesions (8.36![]() 2.63
kPa versus
182.25
2.63
kPa versus
182.25![]() 55.88
kPa for human). Our study highlights that such
differences in
the distribution and amplitude of vessel wall stress might be one key
feature
for explaining for the difference in lesion stability between human
coronary
and mouse aortic lesions.
55.88
kPa for human). Our study highlights that such
differences in
the distribution and amplitude of vessel wall stress might be one key
feature
for explaining for the difference in lesion stability between human
coronary
and mouse aortic lesions.
|
|
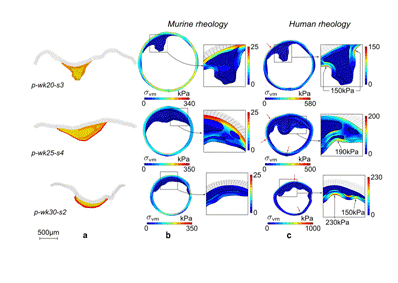
Fig. 1: Influence of the mechanical properties (MP) of the atherosclerotic vessel on the in vivo stress distributions. Finite-Element (FE) simulations were performed for 3 atherosclerotic mouse lesions. Column a: Zero-stress configurations of pathological samples # 3, 4 and 2 for mice of 20, 25 and 30 weeks, respectively (yellow: LiRi regions, orange: CeFb regions, red: HyFb regions, white: arterial wall). Column b) In vivo stress distributions computed by considering the mouse MP. Column c) In vivo stress distributions computed by considering the human MP. A pressure of 14.5 kPa was used for these FE simulations. Red arrows indicate regions with higher stresses. |
Selected Publications
• Ohayon J, Mesnier N, Broisat A, Toczek J, Riou L, Tracqui
P. Elucidating atherosclertic
vulnerable plaque rupture by modeling
cross substitution of ApoE-/-
mouse and human plaque component stiffnesses.
Biomechanics
and Modeling in Mechanobiology.
(in press), 2011.
• Ohayon J, Gharib AM,
Garcia A, Heroux
J, Yazdani
SK, Malvè
M, Tracqui
P, Martinez MA, Doblare
M, Finet G,
Pettigrew RI. Is arterial wall-strain
stiffening and additional process responsible for atherosclerosis in coronary
bifurcations? in
vivo Study Based on
• Broisat A, Toczek J, Mesnier N, Tracqui P, Ghezzi C, Ohayon J, Riou L.
Assessing the low levels of mechanical stress in aortic atherosclerosis
lesions
from ApoE-/-mouse . Arterioscler
Thromb Vasc Biol. 31(5):1007-10,
2011.
• Tracqui P, Broisat A, Toczek J, Mesnier N, Ohayon J, Riou L. Mapping
elasticity moduli of
atherosclerotic plaque in situ
via atomic force microscopy . Journal of
structural Biology 174(1):115-23,
2011.
• Heroux J, Gharib AM,
• Soloperto
G, Keenan NG, Sheppard MN, Ohayon J,
Wood N, Pennell DJ, Mohiaddin
RH, Xu XY. A combined
imaging, computational and
histological analysis of a ruptured carotid plaque. Artery
Research, 4(2):59-65,
2010.
• Le
Floc'h S, Cloutier G, Finet G,
Tracqui P,
Pettigrew RI, Ohayon J. On the potential
of a new IVUS elasticity modulus imaging approach for detecting
vulnerable
atherosclerotic coronary plaques: in vitro vessel phantom study. Phys.
Med. Biol., 55:5701-5721, 2010.
• Finet G., Huo Y, Riouffol
G, Ohayon J,
Guerin P, Kassab
GS. Structure-function
relation in the coronary
artery tree: from fluid dynamics to arterial bifurcations. EuroIntervention,
6:J10-J15, 2010.
• Le Floc'h
S, Ohayon J, Tracqui P, Finet G, Gharib
AM, Maurice R, Cloutier
G, Pettigrew RI. Vulnerable Atherosclerotic Plaque
Elasticity Reconstruction Based on a Segmentation-Driven Optimization
Procedure
Using Strain Measurements: Theoretical Framework. IEEE
Trans Med Imaging, 28(7):1126-37, 2009.
• Kotys MS, Herzka DA, Vonken EJ, Ohayon J, Heroux J, Gharib AM, Stuber M, Pettigrew
RI. Profile order and time-dependent artifacts
in contrast-enhanced coronary MR angiography at 3T: origin and
prevention.
Magn Reson
Med.,
62(2):292-9, 2009.
• Eskandari H, Salcudean SE, Rohling R, Ohayon J. Viscoelastic
characterization of soft tissue from dynamic finite element models. Physics in
Medicine
and Biology, 53(22):6569-90,
2008.
• Ohayon J, Finet G, Gharib
AM, Herzka DA, Tracqui P, Heroux J, Rioufol G, Kotys MS, Elagha A, Pettigrew RI. Necrotic
core thickness asnd
positive arterial remodeling
index: emergent biomechanical factors for evaluating the risk of plaque
rupture. Am J Physiol Heart Circ Physiol.,
295(2):H717-27, 2008.
• Ohayon J, Dubreuil O, Tracqui P, Le Floc'h S, Rioufol G, Chalabreysse L, Thivolet F,
• Boudou T.,
Ohayon J., Arntz
Y., Finet G., Picart C., Tracqui P. An extended modeling
of the micropipette
aspiration experiment for the characterization of the Young’s
modulus and
Poisson’s ratio of adherent thin biological samples:
Numerical and experimental
studies. Journal of Biomechanics, 39:1677-85, 2006.
• Boudou T., Ohayon J., Picart C., Tracqui P. Characterization of the Young’s modulus and Poisson’s ratio of polyacrylamide gels using micropipette aspiration technique. Biorheology, 43(6): 721-8, 2006.