Imaging in vivo the Atherosclerotic Vulnerable Plaque
Jacques Ohayon, PhD and Simon Le Floc’h, PhD
Laboratory TIMC-IMAG/DyCTiM, UJF,
CNRS UMR 5525, In3S
Faculty of Medicine of Grenoble - 38706 La Tronche Cedex, France
Email: Jacques.Ohayon@imag.fr
On the potential of a new IVUS elasticity modulus imaging approach for detecting vulnerable atherosclerotic coronary plaques: in vitro vessel phantom study - Physics in. Medicine and Biology, 55:5701-5721, 2010 - Peak cap stress amplitude is recognized as a good indicator of vulnerable plaque (VP) rupture. However, such stress evaluation strongly relies on a precise, but still lacking, knowledge of the mechanical properties exhibited by the plaque components. As a first response to this limitation, our group recently developed, in a previous theoretical study, an original approach, called iMOD, which reconstructs elasticity maps (or modulograms) of atheroma plaques from the estimation of strain fields. In the present in vitro experimental study, conducted on PVA-C arterial phantoms, we investigate the benefit of coupling the iMOD procedure with the acquisition of intravascular ultrasound (IVUS) measurements for detection of VP. Our results show that the combined iMOD-IVUS strategy : 1) successfully detected and quantified soft inclusion contours with high positive predictive values and sensitivities of 89.7 ± 3.9% and 81.5 ± 8.8 %, respectively, 2) estimated reasonably cap thicknesses larger than ~300 µm, but underestimated thinner caps, and 3) quantified satisfactorily Young's modulus of hard medium (mean value of 109.7 ± 23.7 kPa instead of 145.4 ± 31.8 kPa), but overestimated the stiffness of soft inclusions (mean Young`s moduli of 31.4 ± 9.7 kPa instead of 17.6 ± 3.4 kPa). All together, these results demonstrate a promising benefit of the new iMOD-IVUS clinical imaging method for in vivo VP detection.
|
|
|
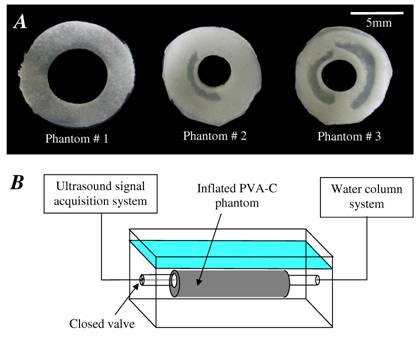
Fig. 1: (A) Sections of the three PVA cryogel phantoms. (B) Schematic draw of the experimental setup composed of a water tank, the PVA-C phantom, a water column system to pressurize the phantom and the ultrasound acquisition system. |
|
|
|
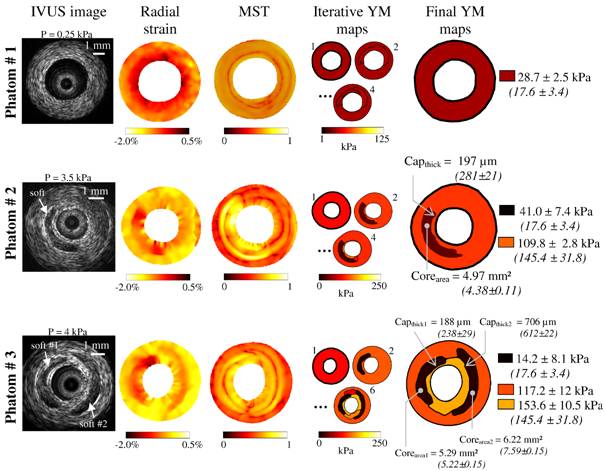
Fig. 2: Performance of the LSME-iMOD method to detect soft inclusions using experimental IVUS images acquired on the three PVA-C phantoms presented in Fig. 1. Column 1: IVUS images. Column 2: Estimated radial strain fields obtained by using the Lagrangian Speckle Model Estimator (LSME). Such strain fields result from pressure loading of 0.25 kPa, 3.5 kPa and 4 kPa, for phantoms # 1, 2 and 3, respectively. Column 3: Spatial pseudo-gradient elasticity field resulting from the modified Sumi’s transform (MST) procedure. Column 4: Evolution of the Young’s modulus (YM) map obtained during the iterative procedure. Column 5: Final Young’s modulus maps and resulting estimations of cap-thicknesses (Capthick) and necrotic cores areas (Corearea). In parenthesizes are given the real values measured by histomorphometry (gold standard values). |
Vulnerable Atherosclerotic Plaque Elasticity Reconstruction Based on Coupling Dynamical Segmentation with Optimization of Strain Measurements - IEEE Trans Med Imaging, 28(7):1126-37, 2009 - It is now recognized that prediction of the vulnerable coronary plaque rupture requires not only an accurate quantification of fibrous cap thickness and necrotic core morphology but also a precise knowledge of the mechanical properties of plaque components. Indeed, such knowledge would allow a precise evaluation of the peak cap-stress amplitude, which is known to be a good biomechanical predictor of plaque rupture. Several studies have been performed and methods proposed to estimate vascular elasticity. It seems that the main issue for improving such methods does not rely on the optimization algorithm itself, but rather on pre-conditioning requiring the best estimation of the plaque components’ contours. The present theoretical study was therefore designed to develop a pre-conditioning model to extract the plaque morphology in order to initiate the optimization process, and an approach coupling a dynamical watershed segmentation method with the optimization procedure to highlight the modulogram of the atherosclerotic plaque. This combined methodology, based on the continuum mechanics theory prescribing the strain field, was successfully tested on different IVUS coronary lesion images.
|
|
|
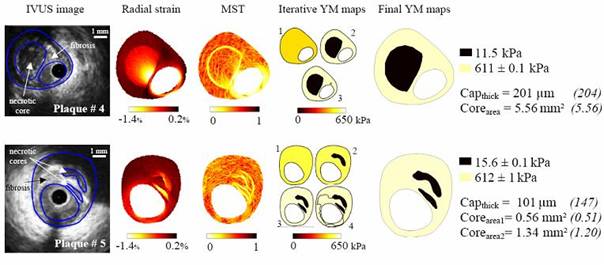
Fig. 3: Performance of our algorithm to detect vulnerable plaques. Two plaques with multiple necrotic cores were used for these simulations. Column (1): IVUS images with plaque morphologies. Column (2): Perturbed radial strain fields obtained with blood pressure of 1 kPa and a default white noise. Column (3): Spatial derivative fields of the Young’s modulus resulting from the Modified Sumi’s Transform (MST) procedure. Column (4): Evolution of the Young’s modulus (YM) map obtained during the application of the combined dynamical Watershed Segmentation and optimisation procedure (DWS-O). Column (5): Final Young’s Modulus maps and resulting estimations of cap-thickness (Capthick) and necrotic cores areas (Corearea). Target values are given in parenthesis, and the targeted fibrosis and the necrotic cores Young’s moduli were 600 kPa and 10 kPa, respectively. |
Selected Publications
• Ohayon J, Mesnier N, Broisat A, Toczek J, Riou L, Tracqui P. Elucidating atherosclertic vulnerable plaque rupture by modeling cross substitution of ApoE-/- mouse and human plaque component stiffnesses. Biomechanics and Modeling in Mechanobiology. (in press), 2011.
• Ohayon J, Gharib AM, Garcia A, Heroux J, Yazdani SK, Malvè M, Tracqui P, Martinez MA, Doblare M, Finet G, Pettigrew RI. Is arterial wall-strain stiffening and additional process responsible for atherosclerosis in coronary bifurcations? in vivo Study Based on Dynamic CT and MRI. Am J Physiol Heart Circ Physiol. 301(3):H1097-106, 2011.
• Broisat A, Toczek J, Mesnier N, Tracqui P, Ghezzi C, Ohayon J, Riou L. Assessing the low levels of mechanical stress in aortic atherosclerosis lesions from ApoE-/-mouse . Arterioscler Thromb Vasc Biol. 31(5):1007-10, 2011.
• Tracqui P, Broisat A, Toczek J, Mesnier N, Ohayon J, Riou L. Mapping elasticity moduli of atherosclerotic plaque in situ via atomic force microscopy . Journal of structural Biology 174(1):115-23, 2011.
• Heroux J, Gharib AM, Danthi NS, Cecchini S, Ohayon J, Pettigrew RI. High Affinity avb3 Integrin Targeted Optical Probe as a New Imaging Biomarker for Early Atherosclerosis: Initial Studies in Watanabe Rabbits. Mol Imaging Biol., 12(1):2-8, 2010.
• Soloperto G, Keenan NG, Sheppard MN, Ohayon J, Wood N, Pennell DJ, Mohiaddin RH, Xu XY. A combined imaging, computational and histological analysis of a ruptured carotid plaque. Artery Research, 4(2):59-65, 2010.
• Le Floc'h S, Cloutier G, Finet G, Tracqui P, Pettigrew RI, Ohayon J. On the potential of a new IVUS elasticity modulus imaging approach for detecting vulnerable atherosclerotic coronary plaques: in vitro vessel phantom study. Phys. Med. Biol., 55:5701-5721, 2010.
• Finet G., Huo Y, Riouffol G, Ohayon J, Guerin P, Kassab GS. Structure-function relation in the coronary artery tree: from fluid dynamics to arterial bifurcations. EuroIntervention, 6:J10-J15, 2010.
• Le Floc'h S, Ohayon J, Tracqui P, Finet G, Gharib AM, Maurice R, Cloutier G, Pettigrew RI. Vulnerable Atherosclerotic Plaque Elasticity Reconstruction Based on a Segmentation-Driven Optimization Procedure Using Strain Measurements: Theoretical Framework. IEEE Trans Med Imaging, 28(7):1126-37, 2009.
• Kotys MS, Herzka DA, Vonken EJ, Ohayon J, Heroux J, Gharib AM, Stuber M, Pettigrew RI. Profile order and time-dependent artifacts in contrast-enhanced coronary MR angiography at 3T: origin and prevention. Magn Reson Med., 62(2):292-9, 2009.
• Eskandari H, Salcudean SE, Rohling R, Ohayon J. Viscoelastic characterization of soft tissue from dynamic finite element models. Physics in Medicine and Biology, 53(22):6569-90, 2008.
• Ohayon J, Finet G, Gharib AM, Herzka DA, Tracqui P, Heroux J, Rioufol G, Kotys MS, Elagha A, Pettigrew RI. Necrotic core thickness asnd positive arterial remodeling index: emergent biomechanical factors for evaluating the risk of plaque rupture. Am J Physiol Heart Circ Physiol., 295(2):H717-27, 2008.
• Ohayon J, Dubreuil O, Tracqui P, Le Floc'h S, Rioufol G, Chalabreysse L, Thivolet F, Pettigrew RI, Finet G. Influence of residual stress/strain on the biomechanical stability of vulnerable coronary plaques: potential impact for evaluating the risk of plaque rupture. Am J Physiol Heart Circ Physiol., 293(3):H1987-96, 2007.
• Boudou T., Ohayon J., Arntz Y., Finet G., Picart C., Tracqui P. An extended modeling of the micropipette aspiration experiment for the characterization of the Young’s modulus and Poisson’s ratio of adherent thin biological samples: Numerical and experimental studies. Journal of Biomechanics, 39:1677-85, 2006.
• Boudou T., Ohayon J., Picart C., Tracqui P. Characterization of the Young’s modulus and Poisson’s ratio of polyacrylamide gels using micropipette aspiration technique. Biorheology, 43(6): 721-8, 2006.